ऑनडेंसट्रॉन टैबलेट 4 मिलीग्राम
उत्पाद विवरण:
- दवा का प्रकार जेनेरिक ड्रग्स
- भौतिक रूप टैबलेट्स
- खुराक डॉक्टर और फोनीशियन के अनुसार
- खुराक संबंधी दिशा-निर्देश डॉक्टर और फोनीशियन के अनुसार
- स्टोरेज निर्देश सूखी और ठंडी जगह
- अधिक देखने के लिए क्लिक करें
ऑनडेंसट्रॉन टैबलेट 4 मिलीग्राम मूल्य और मात्रा
- 1000
- बॉक्स/बॉक्स
- बॉक्स/बॉक्स
ऑनडेंसट्रॉन टैबलेट 4 मिलीग्राम उत्पाद की विशेषताएं
- डॉक्टर और फोनीशियन के अनुसार
- जेनेरिक ड्रग्स
- टैबलेट्स
- डॉक्टर और फोनीशियन के अनुसार
- सूखी और ठंडी जगह
ऑनडेंसट्रॉन टैबलेट 4 मिलीग्राम व्यापार सूचना
- 1000 प्रति दिन
- 15 दिन
- Yes
- हमारी नमूना नीति के बारे में जानकारी के लिए हमसे संपर्क करें
- ऑल इंडिया
उत्पाद वर्णन
Ondansetron tablets :
Healthy Incorporation and Healthy life pharma Pvt ltd are one of the leading manufacturer, supplier, and exporter of Ondansetron tablets 4 mg, Ondansetron tablets 8mg, Ondansetron Orally Disintegrating tablets 4mg,in India and serving the world with best quality pharmaceutical products.
Healthy incorporation and Healthy life pharma Pvt ltd, is Mumbai Based WHO GMP certified manufacturer of injection, tablets, capsules, liquid, dry syrups, pre-filled syringes, ointments etc. with an extensive experience of over 45 years in manufacturing and have reach in many countries. We are committed to provide a stock of highly effective Ondansetron tablets to our innumerable clients with the help of our well-experienced pharmaceutical specialists.
Uses of Ondansetron tablets :
Ondansetron tablets is used alone or in combination with other medications to prevent nausea and vomiting caused by cancer drug treatment i.e., chemotherapy and radiation therapy. It can be used to prevent and cure nausea and vomiting after any surgery. This tablet works by blocking one of the body s natural substances known as serotonin which causes vomiting. Ondansetron tablet belongs to a class of drugs called serotonin 5-HT3 receptor antagonists. It is advisable to take first dose of ondansetron tablet 30 minutes before the start of chemotherapy, or 1 hour before any surgery or 1 to 2 hours before starting radiation therapy. Extra doses are sometimes taken for more than 1 or 2 days after the end of treatment of chemotherapy or radiation therapy.
This tablet is available only on doctor prescription.
Additional Information of Ondansetron tablets:
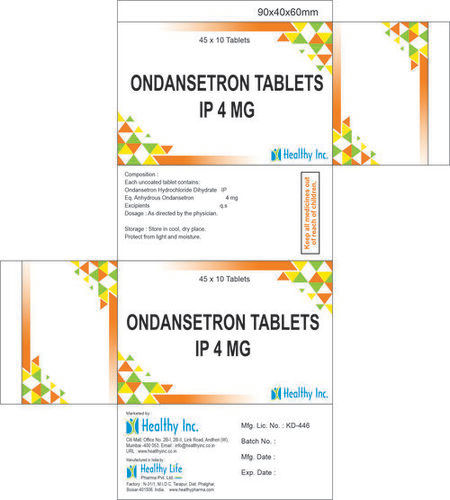
Product Name Ondansetron tablets
Composition & Active ingredients Ondansetron
Potency 4 mg , 8mg , Ondansetron Orally Disintegrating tablets 4mg
Therapeutic use Nausea/vomiting
Packing 10 Tablets (1 Box)
Dosage As per Doctor Prescription
Precautions of Ondansetron tablets:
Please inform doctor your medical history of liver disease, kidney disease, heart disease, stomach problems, QT prolongation, and electrolyte imbalance. Consult your doctor if you are pregnant or planning to get pregnant, or breast- feeding mother.
Side Effects of Ondansetron tablets:
Please note if your doctor has prescribed Ondansetron tablets to you, it means he or she has judged that the benefit to you is big than the risk of side effects. Most of the time there is no serious side effects. Some common side effects may arise after starting medication, as your body adjust to medicine. If any of your symptoms worsen then consult your doctor immediately
- Nausea
- Constipation
- Headache
- Weakness
- Chills
- Drowsiness
- Dizziness
- Rash
- Slow or irregular heartbeat
- Hallucinations
- Chest pain
- Fever
- Vomiting
- Difficulty in breathing
- Confusion
- Excessive sweating
For Detailed Product Information संपर्क करें at +91 7710003340, If you need Any assistance in selecting our Products that fits your requirements. If you re looking for any product that you re not seeing here, please contact our support team.
Note: Healthy Incorporation and Healthy life pharma Pvt ltd tries to ensure that all information, whether in relation to the products, services, offerings provided as part of this website is correct at the time of inclusion on the website, Unauthorised use of any materials contained on this website may violate copyright laws, trademark laws, the laws of privacy and publicity, certain communications statutes and regulations and other applicable laws and regulations. All copyright and other intellectual property rights in this material are owned by Healthy Incorporation and Healthy life pharma Pvt ltd. Please connect with us to discuss precise product specifications and requirements and obtain advice on which products are suitable for your requirement or you can write to us info@healthyinc.co.in

Price: Â
- 50
- 100
- 200
- 250
- 500
- 1000+
Common Medicines & Drugs अन्य उत्पाद
 |
HEALTHY INC.
सर्वाधिकार सुरक्षित.(उपयोग की शर्तें) इन्फोकॉम नेटवर्क प्राइवेट लिमिटेड . द्वारा विकसित एवं प्रबंधित |













 जांच भेजें
जांच भेजें एसएमएस भेजें
एसएमएस भेजें